While unmyelinated sensory afferent C fibers are classically considered to code for painful stimuli and tissue status, skin C-fiber low-threshold mechanoreceptors (C-LTMRs) respond instead to innocuous tactile stimuli and are uniquely tuned to transmit information about pleasant affiliative touch. They respond well to gentle moving stimuli so that their firing rates correlate significantly with stimulus pleasantness ratings. Unlike the myelinated mechanosensory pathway that project to somatosensory cortex, C-LTMRs in humans – where they are called C-tactile or CT afferents – relay information to the insula, an area associated with allostatic control, motivation, and feelings.
We hypothesize that SCI transforms these pleasant touch-encoding C-LTMRs into allodynia-encoding nociceptors in body regions associated with the segmental site of injury. To test this hypothesis we have developed an ex-vivo skin-nerve preparation to characterize the properties of C-LTMRs, first by their selective optogenetic activation, then in response to mechanical brush stimuli applied at a range of velocities within preferred forces. Our collaborator, Dr. Sandra Garraway, is undertaking important complementary studies in vivo.

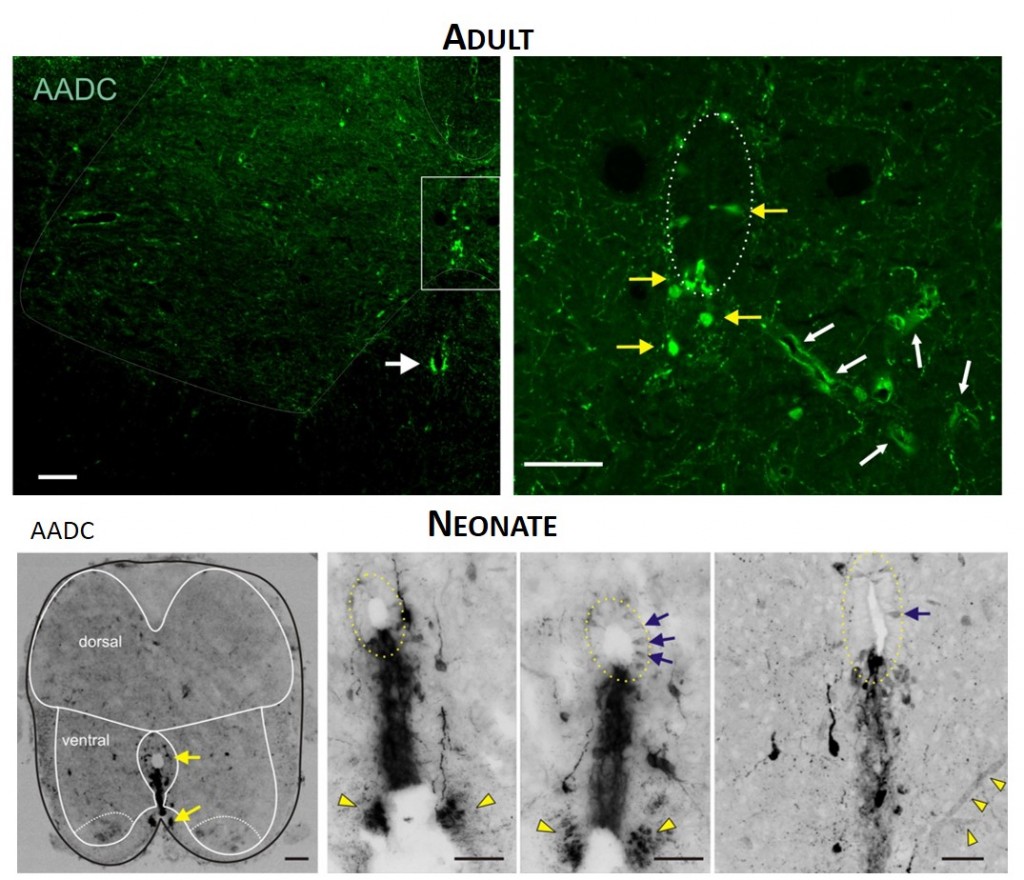
 TOP. LEFT. Intense AADC immunolabeling is found in blood vessels, in the D cells and in the ventromedial white matter (white arrow in left panel). Note that some AADC+ neurons are also found in the dorsal central canal. RIGHT. Higher power image of central canal region (outlined). Blood vessels are identified with white arrows and AADC+ neurons with yellow arrows. BOTTOM. LEFT. Low power transverse spinal cord section (10 µm) of AADC immunolabeling. Superimposed is an outline of the spinal cord (black) with interior white lines approximately identifying dorsal and ventral gray matter and central canal region. Note strongest labeling is associated with D cells intermingled with epithelial cells surrounding the central canal (top arrow). Also, note the associated vertical row of cellular labeling projecting ventrally, and bilateral white matter labeling in the ventral funiculus (bottom arrow). RIGHT. Three panels showing higher power images from separate sections illustrate the diversity of AADC labeling in the ventral medial grey matter region. Epithelial cell layer surrounding central canal is outlined while blue arrows point to example D cells. Common to all is the vertical stream of projections with intermingled cells ventral to the central canal. These appear to end in a white matter tract in the ventral funiculus (yellow arrowheads).
TOP. LEFT. Intense AADC immunolabeling is found in blood vessels, in the D cells and in the ventromedial white matter (white arrow in left panel). Note that some AADC+ neurons are also found in the dorsal central canal. RIGHT. Higher power image of central canal region (outlined). Blood vessels are identified with white arrows and AADC+ neurons with yellow arrows. BOTTOM. LEFT. Low power transverse spinal cord section (10 µm) of AADC immunolabeling. Superimposed is an outline of the spinal cord (black) with interior white lines approximately identifying dorsal and ventral gray matter and central canal region. Note strongest labeling is associated with D cells intermingled with epithelial cells surrounding the central canal (top arrow). Also, note the associated vertical row of cellular labeling projecting ventrally, and bilateral white matter labeling in the ventral funiculus (bottom arrow). RIGHT. Three panels showing higher power images from separate sections illustrate the diversity of AADC labeling in the ventral medial grey matter region. Epithelial cell layer surrounding central canal is outlined while blue arrows point to example D cells. Common to all is the vertical stream of projections with intermingled cells ventral to the central canal. These appear to end in a white matter tract in the ventral funiculus (yellow arrowheads).